BioAgile Therapeutics
#1 Clinical Research
Organization (CRO)
In India


INOVATE | EXECUTE | DELIVER
ISO Certified Contract Research Organization
At BioAgile Therapeutics, we deliver innovative clinical research solutions with a commitment to quality, compliance, and client success.
The mission of BioAgile Therapeutics is to be the leading Clinical Research Organization and to build value for our clients, partners and their stakeholders.
BioAgile caters business solutions to Pharmaceuticals, Nutraceuticals, Herbs, Cosmetics and Medical Devices industries. The clinical research organization built by a dedicated team having diverse talents and expertise to provide clinical services in the most ethical ways.
We offer Clinical Research Services comprising of Medical Writing, Clinical Data Management, Site Management, Biostatistical and Regulatory Services making us boutique CRO (Clinical Research Organization) offering an end to end services with an international reach, combined with the level of quality and customer service.


Delivering Excellence in Clinical Research for Your Success
Our Clinical Research Services
We provide end-to-end clinical research solutions with a focus on quality, compliance, and innovation, ensuring reliable results and regulatory success.
















Top Clinical Research Services In India
Why Choose BioAgile Therapeutics?
BioAgile Therapeutics is a trusted partner in clinical research, offering end-to-end solutions with a focus on quality, compliance, and innovation.
Full-Service Expertise
Specialized Experience
Responsive Support

Your Path to Regulatory-Ready Clinical Success Starts Here
Our Engagement Process
All BioAgile partnerships are anchored in transparency, regulatory rigor, and scientific precision ensuring smooth project execution from initiation to submission.
01. Submit a Project Brief
02. Regulatory & Scientific Assessment
03. Customize Your Project Scope
04. Launch with BioAgile Experts
Industries We Serve
BioAgile Therapeutics specializes in clinical research solutions for pharmaceuticals, nutraceuticals, medical devices, and healthcare industries in India. Partner with us for expert services that accelerate innovation and ensure regulatory compliance.
Cosmeceuticals
SaMD (Software as a Medical Device)
Fast-Moving Consumer Goods (FMCG)
Ayurvedic & Herbals
Biotechnology


Customer Testimonials
Customer Satisfaction is Our Working Motivation!
Top Rated
Director, Business Development
CSO
Divya Chandradhara, a Biochemist by education is currently the CEO of BioAgile Therapeutics. Ms. Divya is having several years of experience in Clinical & Pre clinical research industry. Prior to joining BioAgile Therapeutics, Divya has been associated with Advinus Therapeutics, TATA group handling business for domestic and International market. She is a well known person in the industry having collaborations with various MNC’s and academic institutions. Divya holds several international publications in the clinical studies/ pre – clinical studies, and all of them cited internationally.

Mrs. Divya C. - About
CEO BioAgile Therapeutics
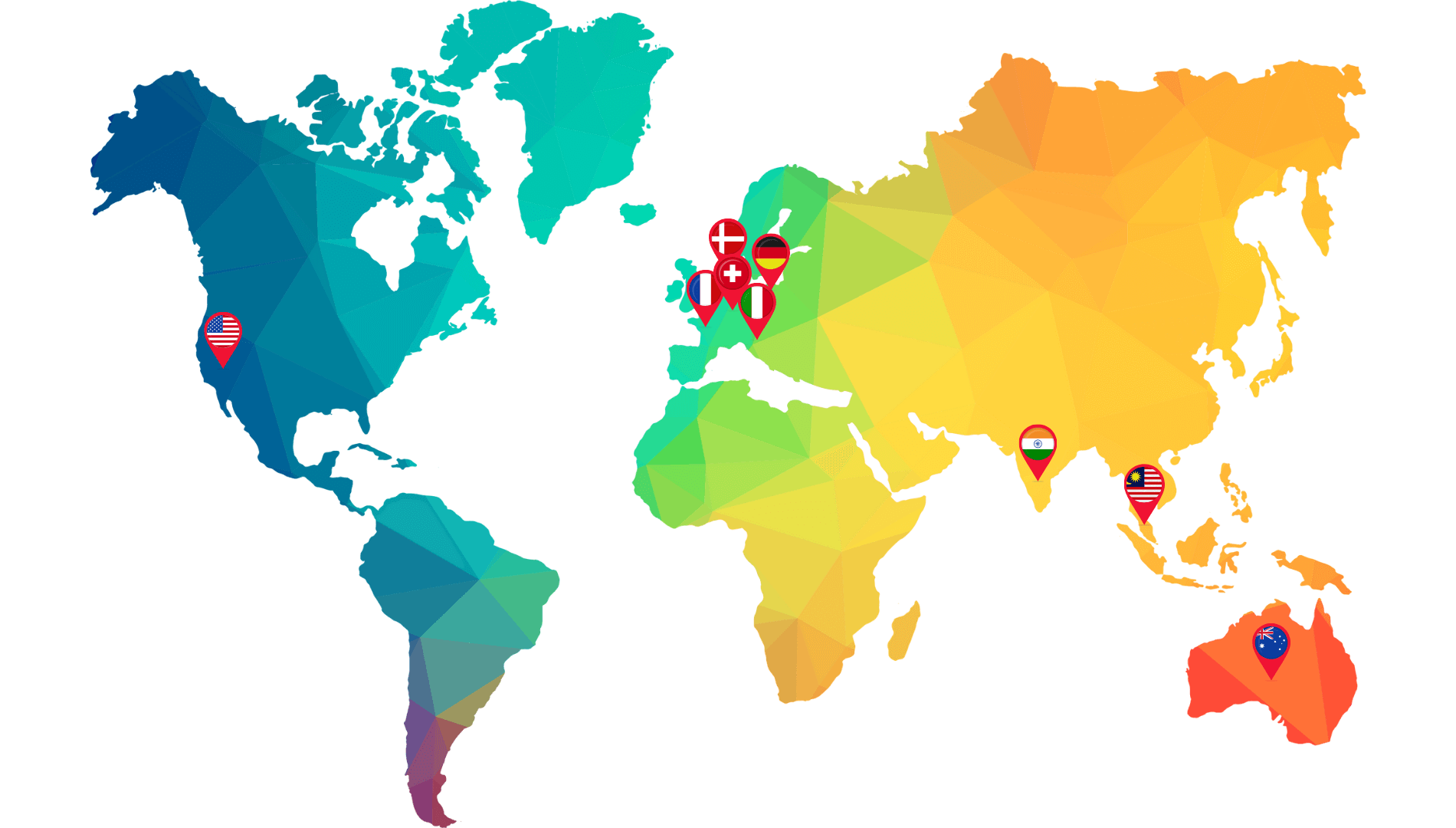
Partner with India’s agile, full-service CRO for compliant, customized, and cost-effective research solutions.
Accelerate Your Product to Market With Confidence
Why BioAgile Therapeutics? Insight-Driven. Results-Focused. Globally Compliant.
Frequently Asked Questions.
Curious about our clinical research process, timelines, or regulatory standards?
Explore answers to the most common queries from sponsors, partners, and investigators.
End-to-End Clinical Research Solutions
Accelerate development with scalable, ICH-GCP–aligned services across the drug, device, and supplement lifecycle.
Begin your
Journey with BioAgile!
Whether you’re starting your career or bringing years of experience, grow with BioAgile – India’s fastest-growing clinical research company. Join a workplace that values your contribution and offers continuous learning and growth.







